The Benefit-Risk Evaluation Challenge
Pharmaceutical teams face mounting pressure to demonstrate drug safety and efficacy across every stage, from regulatory submission to market access and post approval surveillance. Yet traditional evaluation methods create critical vulnerabilities.
Time-Consuming Manual Analysis
Full benefit-risk assessments across the drug lifecycle can span 12-18 months, while individual PSUR/PBRER cycles alone take 12-16 weeks. Each cycle is a manual, resource-intensive process that delays critical go/no-go decisions and market authorization filings.
Incomplete Evidence Synthesis
Fragmented data sources and manual literature reviews miss critical safety signals, leaving gaps in regulatory submissions and HTA dossiers.
High Failure Costs
With 9 out of 10 drugs failing (57% efficacy issues, 17% safety concerns) at an average cost of $2.3B per approval (Source: Tufts CSDD, 2020), incomplete benefit-risk intelligence compounds financial risk across your entire portfolio.
One Platform.
Complete Visibility.
From Clinical Framing to Automated Outputs: six Integrated Stages for Comprehensive Benefit-Risk Intelligence.
100B+ data points | 24 AI models | 20+ clients | pre-filled benefit-risk analysis
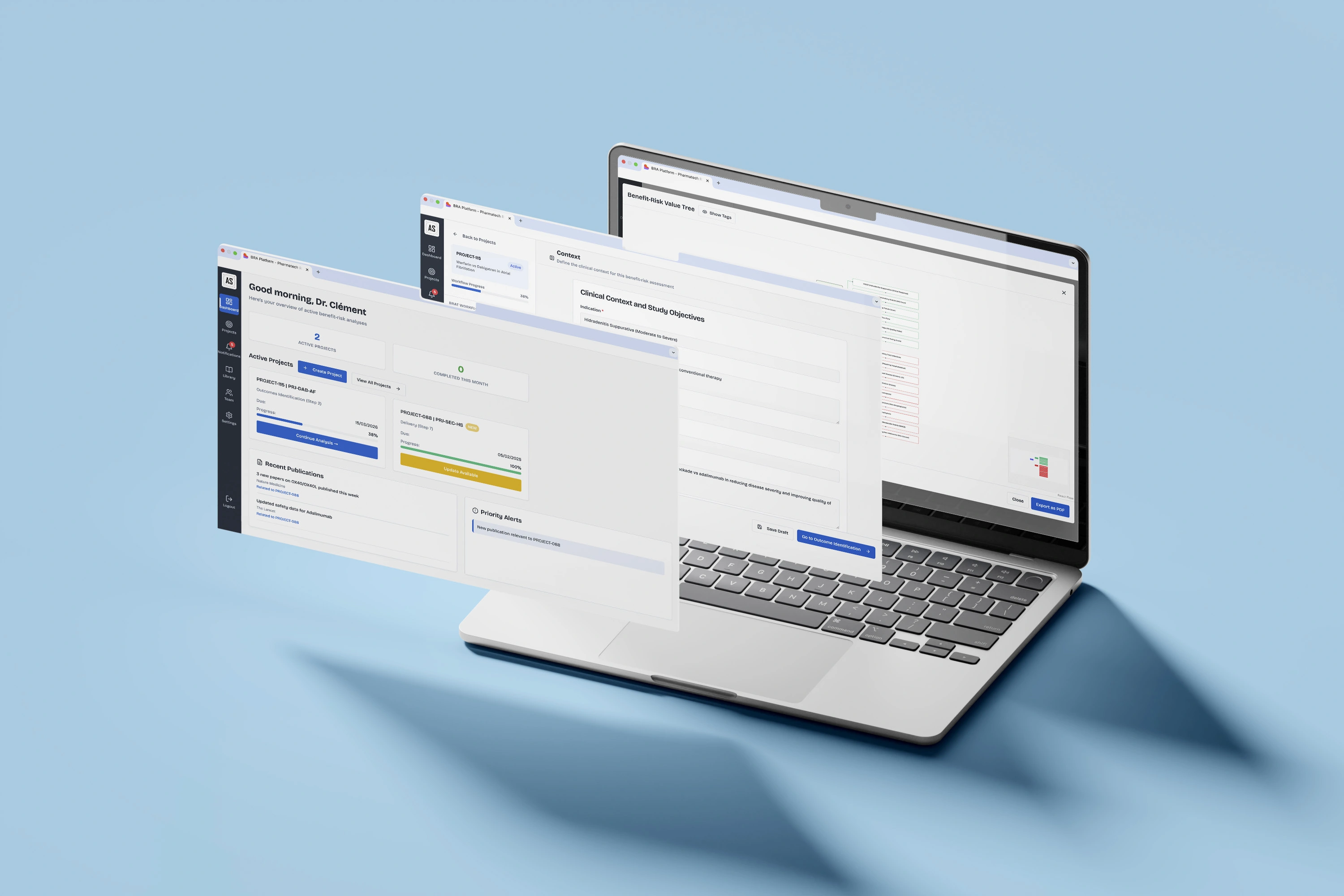
Clinical Framing
Set protocol parameters (drug, dosage, comparator), establish key endpoints and decision criteria, and frame your regulatory strategy from day one: ensuring alignment across clinical, regulatory, and market access teams.
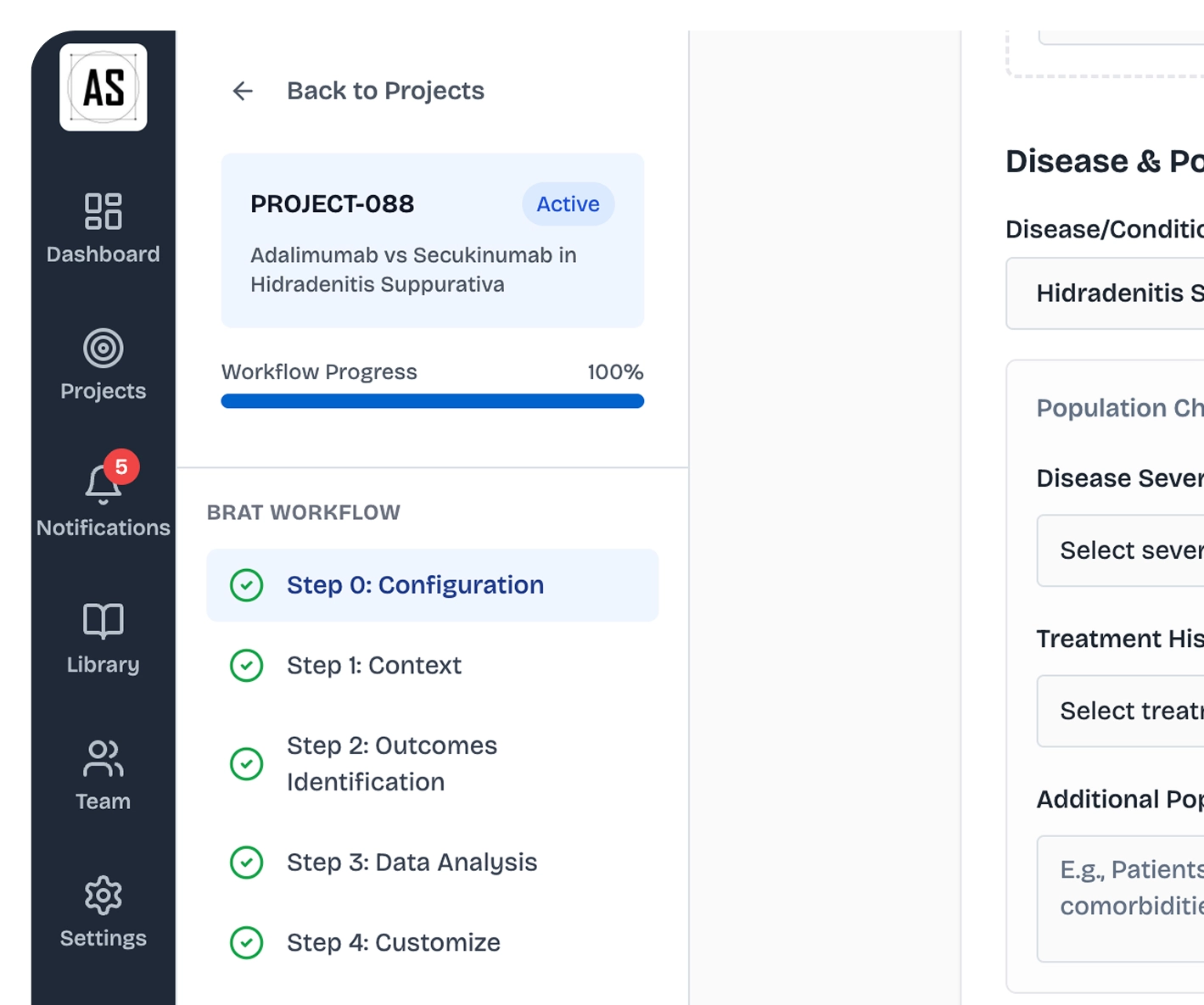

Building the world's largest benefit-risk intelligence database
Once your framework is defined, 24 proprietary AI models process millions of data points from clinical trials, RWE, and regulatory submissions: identifying thousands of risk signals across your portfolio. Our Benefit-Risk Triage Model (BRTM) then validates these insights, isolating key risks and benefits for regulatory decisions.
AI-Powered Data Processing
Our 24 clinician-trained AI models process your clinical trial data alongside the world's largest benefit-risk database (100+ billion data points from RWE, scientific literature, and regulatory submissions). What used to take 18 months now completes in seconds.
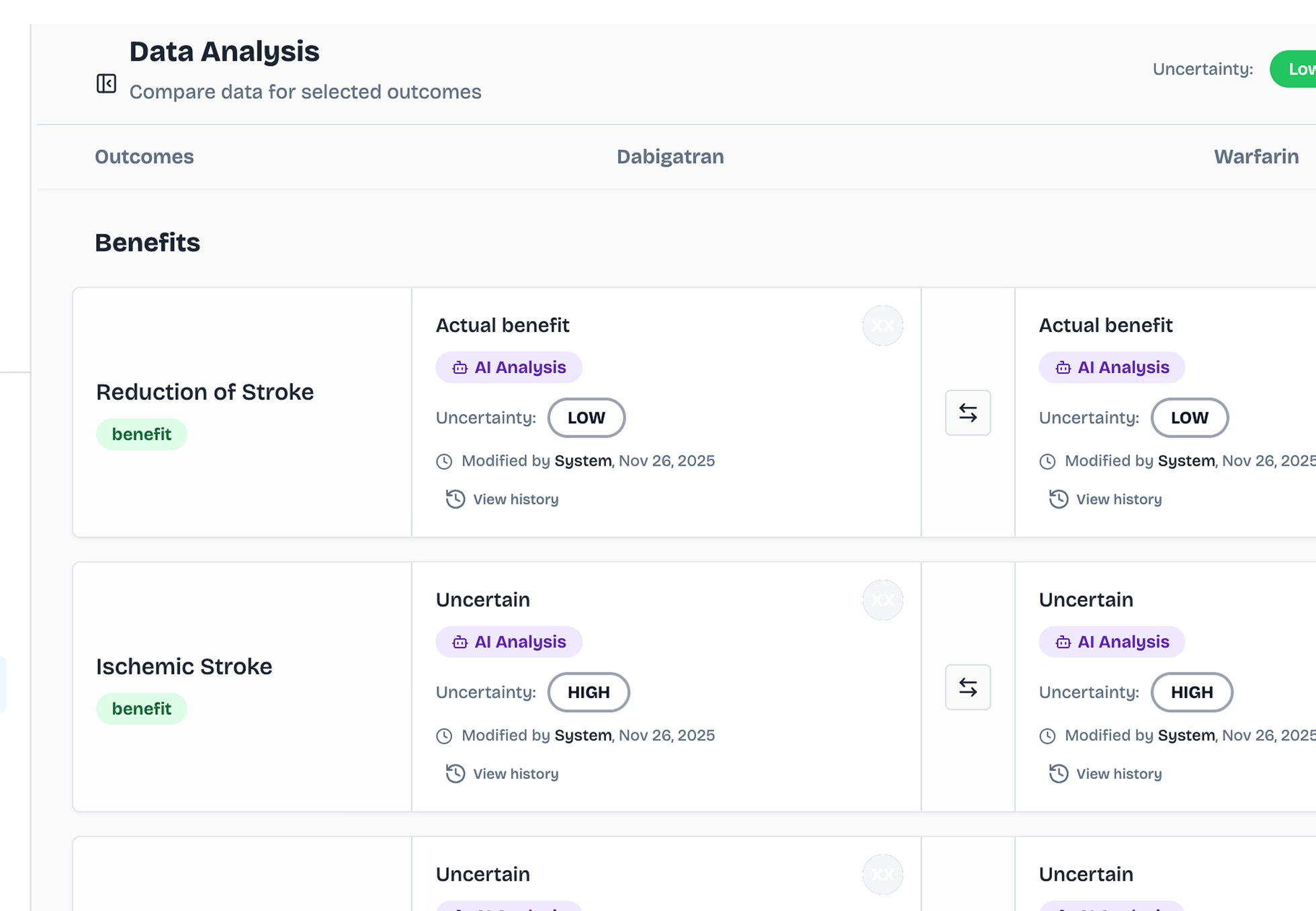
Strategic Analysis
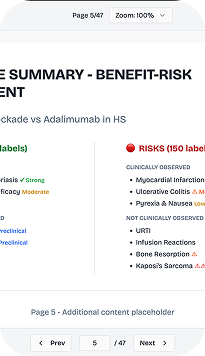
Pinpoint Key Benefits and Key Risks tailored to your submission strategy. Compare against competitors with precision, and build evidence-based arguments that align with regulatory expectations and HTA frameworks.
Decision Intelligence
Focus on the endpoints that drive regulatory decisions. Track safety signals in real-time as new evidence emerges, and adapt your strategy based on post-marketing surveillance data: all within a transparent, auditable dashboard.
"For the first time in our history, we successfully achieved a benefit-risk drill-down prediction based on 9x more insights, anticipating thromboembolic risks and re-routing millions in development funds."
Global R&D Team
Philippe Peyre, Secretary General, Vice President, Sanofi.
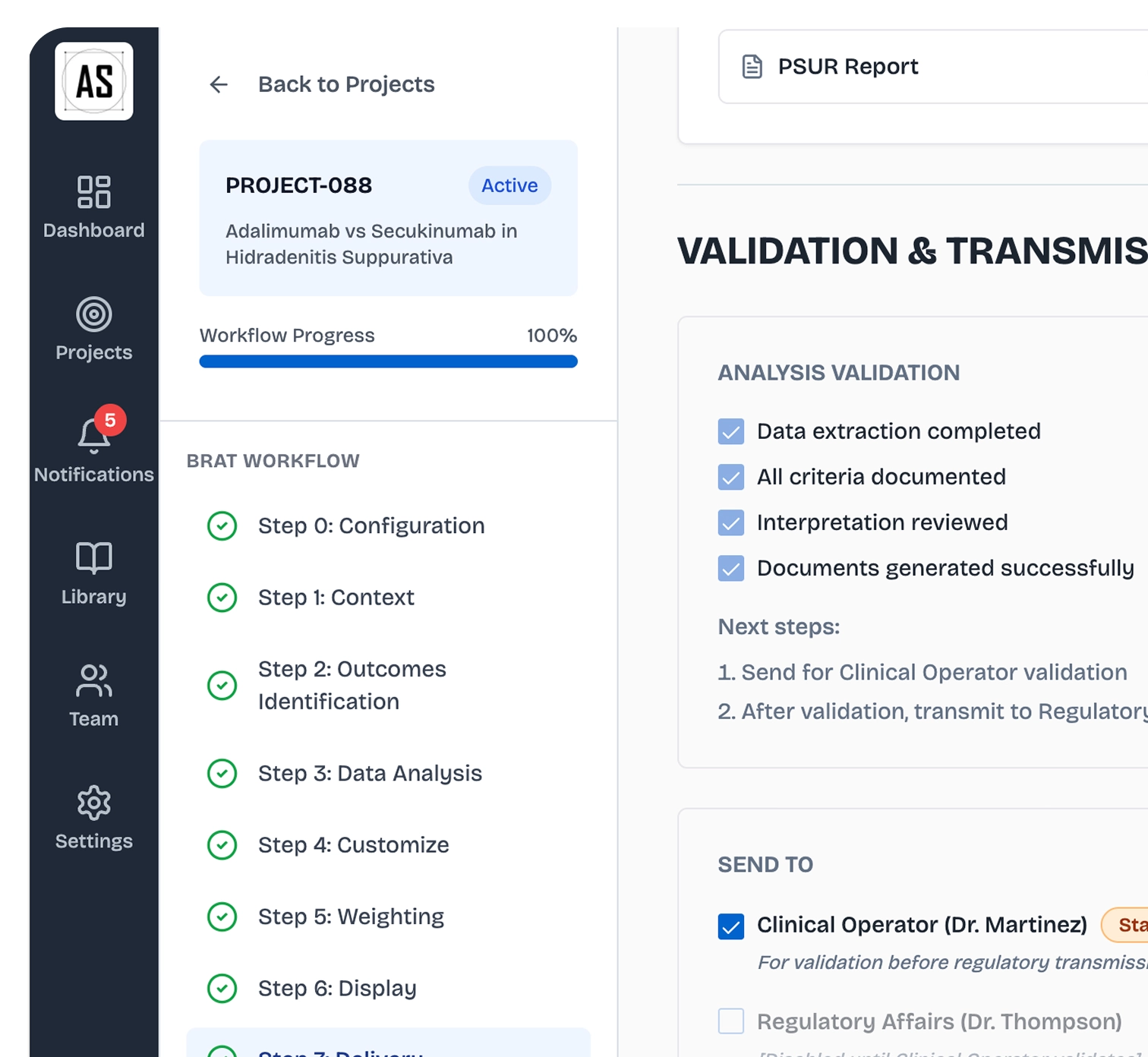
Automated Outputs
Auto-generate study reports, regulatory dossiers, and HTA submissions with full audit trails for compliance. Share analyses across cross-functional teams and maintain version control throughout the lifecycle.




Built by scientists.
Trusted by pharmaceutical leaders.
Founded in 2018 by pharmaceutical and medical experts, ArcaScience bridges cutting edge AI with regulatory science. Our platform supports benefit risk decisions across the entire drug lifecycle, from clinical development through post marketing surveillance.
Our Partners
Trusted by leading pharmaceutical companies, research institutions, and regulatory bodies worldwide.




50+ regulatory submissions supported |
12 therapeutic areas
The Science Behind the Platform
ArcaScience was built to solve a $13 billion market problem: the lack of comprehensive, real-time benefit-risk intelligence. Our mission is to revolutionize drug evaluation and become the world leader in benefit-risk assessment: replacing legacy service models with transparent, AI-powered decision intelligence.

100B+ Data Points
The world's most comprehensive benefit-risk database (AS Profiling Base 100b®), layering 100% of publicly available scientific literature, RWE, and regulatory submissions.
Peer-Reviewed Methodologies
Published research validating our AI ensemble models for phenotyping, safety signal detection, and comparative effectiveness analysis.
Collaborative Partnerships
Working alongside leading research institutions, regulatory bodies, and pharmaceutical innovators to continuously refine our platform with real-world feedback.
Compliance by design
Pharmaceutical data demands the highest standards. ArcaScience is architected from the ground up to meet global regulatory requirements, ensuring your benefit-risk analyses are audit-ready and legally defensible.




Ready to Transform Your Benefit-Risk Evaluations?
Join 20+ pharmaceutical leaders who've cut evaluation time by 60% and costs by 70%. See how ArcaScience delivers complete benefit-risk intelligence: from clinical framing to automated submissions.






